mediated protein degradation pathway in cancer Biology Diagrams The Ubiquitin-Proteasome Pathway. The major pathway of selective protein degradation in eukaryotic cells uses ubiquitin as a marker that targets cytosolic and nuclear proteins for rapid proteolysis (Figure 7.39).Ubiquitin is a 76-amino-acid polypeptide that is highly conserved in all eukaryotes (yeasts, animals, and plants).Proteins are marked for degradation by the attachment of ubiquitin to Ubiquitin‐mediated protein trafficking and localization (by Figdraw). (A) Ubiquitin‐mediated protein degradation in the endoplasmic reticulum (ER) involves tagging misfolded proteins for transport to the proteasome. (B) Ubiquitination facilitates the reorganization of COPII vesicles, allowing them to form larger vesicles capable of

Ubiquitin modifications control a plethora of vital cellular processes through proteolytic and nonproteolytic mechanisms, including proteasomal degradation and proteostasis, selective autophagy

dependent translation control mechanisms: Degradation and ... Biology Diagrams
Role of SCF ubiquitin-ligase and the COP9 signalosome in the N gene-mediated resistance response to Tobacco mosaic virus. Plant Cell 2002; 14 :1483-1496. CAS PubMed PubMed Central Google Scholar

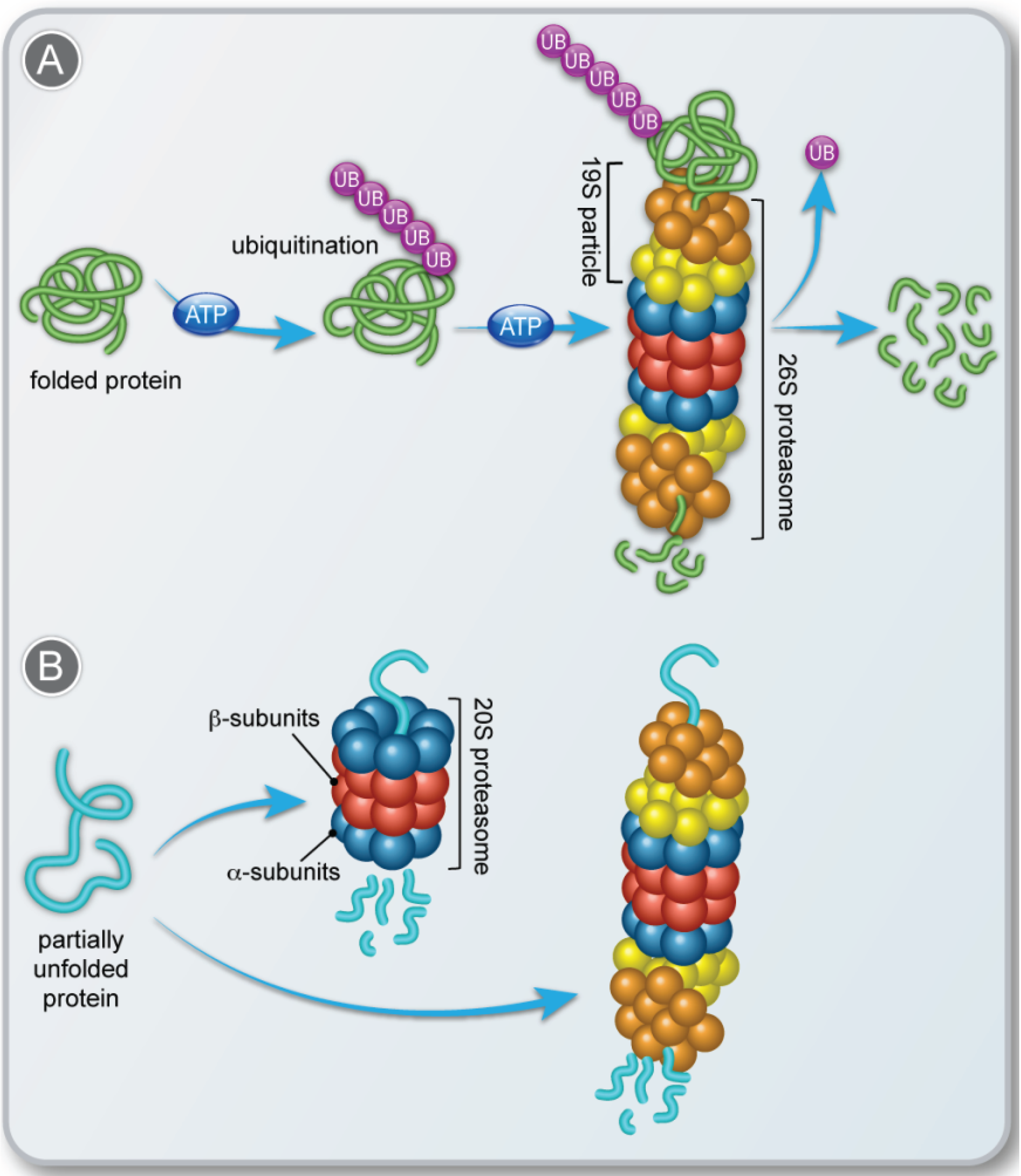
Ubiquitin-mediated degradation is involved in physiological regulation of many cellular processes, including cell cycle progression, differentiation, and signal transduction. Here, we review the basic mechanisms of the ubiquitin system and the various ways in which ubiquitin-mediated degradation can be modulated by physiological signals. In a recent controversy, van Wijk proposed that the vesicle-mediated pathways may be involved in the ubiquitinated degradation of chloroplast proteins, 41 and the CELL DIVISION CYCLE48 (CDC48) dependence of the processing of ubiquitinated chloroplast proteins does point to the existence of an export system. 42 Similarly, our results revealed Ubiquitin-mediated proteasomal degradation is an important mechanism to control protein load in the cells. Ubiquitin binds to a protein on lysine residue and usually promotes its degradation through 26S proteasome system. Abnormal proteins and regulators of many processes, are targeted for degradation by the ubiquitin-proteasome system.
mediated degradation of SlPsbS regulates low night ... Biology Diagrams
Degradation of a protein via the ubiquitin pathway proceeds in two discrete and successive steps: (i) covalent attachment of multiple ubiquitin molecules to the protein substrate, and (ii) degradation of the targeted protein by the 26S proteasome complex with the release of free and reusable ubiquitin. To ensure efficient and specific removal While ZNF598-mediated ubiquitylation is required to rescue stalled and/or collided 80S ribosomes, the surprising outcome of RNF10-mediated ribosome ubiquitylation is degradation of the entire 40S subunit (Figure 3B). 61, 62 Prolonged ubiquitylation of uS3 and uS5 by either RNF10 overexpression or USP10 depletion leads to an approximately 17%
